Page 13 < previous page > <next page>
SOME HEALTH & SCIENCE NEWS
1. Warning about Levothyroxine Drugs
2. Lyme Disease News
3. Dr. Dodds on Urinary Incontinence in Spayed Females
4. Canine Cancer Trial may lead to Human Treatment
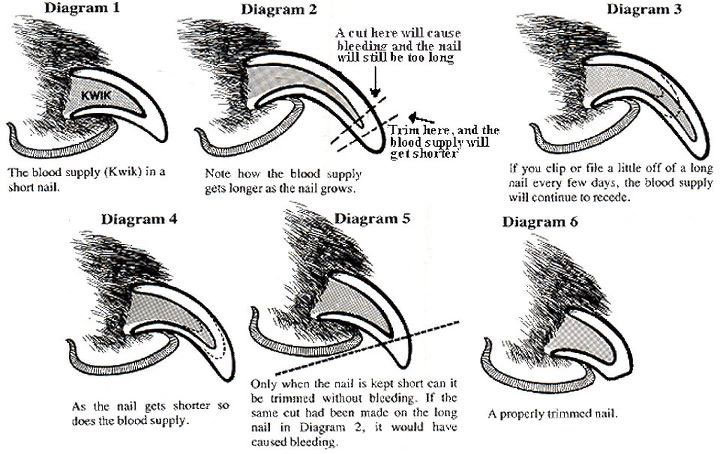
5. Diagram on how to cut nails
FDA Issues Warning Letters to Manufacturers of Unapproved Levothyroxine Drugs for Hypothyroidism in Dogs
FDA
U.S. Food and Drug Administration
Protecting and Promoting Your Health
January 29, 2016
The U.S. Food and Drug Administration has issued warning letters to six manufacturers of unapproved levothyroxine sodium drugs for hypothyroidism in dogs. These unapproved products have not been reviewed by the FDA for safety and effectiveness.
Thyro-Tabs Canine (levothyroxine sodium tablets), (NADA 141-448) is the only FDA-approved drug for replacement therapy for diminished thyroid function (commonly known as hypothyroidism) in dogs.
FDA is concerned about unapproved animal drugs. Animal drugs that have not been reviewed and approved by FDA may not meet FDA’s strict standards for safety and effectiveness. They also may not be properly manufactured or labeled.
FDA issued warning letters to manufacturers of the following unapproved levothyroxine products:
Thyrosyn
Soloxine
Levocrine
Thyromed
Thyroid Chewable Tablets
Thyrokare
Thyroxine L
Thyrovet
Leventa
If the companies that were issued the warning letters continue to market the violative products, they may be subject to enforcement action, including seizure of violative products and/or an injunction.
Shipments of levothyroxine active pharmaceutical ingredient (API) not referenced in an approved animal drug application or the subject of an index listing or a valid investigational use exemption and unapproved finished animal drugs containing levothyroxine offered for import may be subject to refusal.
Dog owners and veterinarians can report to the FDA any adverse events, including ineffectiveness, in dogs that received approved or unapproved hypothyroidism products. Information on reporting adverse events can be found at: http://www.fda.gov/animalveterinary/safetyhealth/reportaproblem/ucm055305.htm.
Warning Letters
Neogen Corporation
Quality Animal Care Manufacturing
Virbac Animal Health, Inc.
Dechra Veterinary Products LLC
Diamond Animal Health Inc.
Merck Animal Health
LYME Disease NEWS
shared by Patty Ewing
INTRODUCING BORRELIA MAYONII, A NEW BORRELIA SPECIES ASSOCIATED WITH HUMAN
LYME BORRELIOSIS IN THE USA, ANDREW ESCHNER, DVM
BRIEF SUMMARY OF FINDINGS:
Research conducted and reported by the MAYO CLINIC and the CDC was recently published in the British Journal, The Lancet (Lancet Infect Disease, 2016). The new species name ‘mayonii’ is named for its discoverer – the Mayo Clinic in Rochester, MN. The new Borrelia species was isolated from approximately 6% of Lyme (+) cases submitted from the upper Midwest USA during a 3 year period starting Jan. 1, 2012. Of 106 (+) Lyme cases, 6 of these were atypical isolates and represented a new species.
Of 66,000 samples tested in the previous 8 year period, NO samples tested (+) for Borrelia mayonii leading researchers to believe that this is a relatively recent occurrence.
FREQUENT DIAGNOSIS FOR A POSSIBLY INFREQUENT CAUSE: URINARY INCONTINENCE IN SPAYED FEMALE
from DR. JEAN DODDS' PET HEALTH RESOURCE BLOG
Is spaying really the cause of leaking urine?
All too often companion dog parents treat urinary incontinence, or uncontrolled or excessive urinating with over-the-counter medications. Don’t misunderstand me, I suggest several of these OTC medications to my clients, but not until a few questions are answered and tests are run. “Self-medicating” your dog simply masks the symptom instead of figuring out the medical condition causing it. Particularly with spayed female dogs, caregivers have been lulled into thinking an ovariohysterectomy (OHE) was the impetus. It makes sense. Some studies indicate that up to 20% of spayed dogs have acquired urinary incontinence (AUI) due to the procedure which may trigger urethral sphincter mechanism incompetence (USMI = a urethra that does not have enough tone to keep the urine from leaking involuntarily).
This begs the question: why do vets continue to perform OHEs if other procedures are available such as laparoscopy, ovariectomy (only ovaries removed) or hysterectomy (uterus removed)? Because we do not know what activates AUI, as OHE may not be the only cause. Regarding the OHE procedure causing USMI, many of my colleagues believe it is caused by the decrease in circulating estrogen that affects urethral tone and some dogs do respond positively to treatment with estrogen products. However, since the estrogen levels in an anestrous, intact female are essentially the same as spayed females and the onset of AUI is generally around three years, another cause may be overlooked during diagnosis. Collagen content has also been questioned but it has been determined that there is no difference in collagen amounts between spayed and non-spayed dogs. Other veterinarians point to the pressure that the reproductive organs place on the bladder to help “seal” it. Ultimately, AUI could be due to multiple factors.
My conclusion: Urinary incontinence attributed to an OHE is often a misdiagnosis. Dogs need to be tested thoroughly to rule out other medical conditions before determining that OHE was the cause.
|
Melbourne canine cancer trial could lead to breakthrough in human treatment, scientists say. The Herald Sun, Melbourne - 15 March 2016 MELBOURNE scientists say a new canine cancer trial has produced exciting results that could lead to a breakthrough in human treatment. Dr Noam Pik, head of the veterinary division at West Melbourne biotechnology research group Biotempus, is leading the free clinical trial and said early results were “mind-blowing”. “If this trial proves effective, we plan to offer this service to canine patients within 24 months and then continue to humans,” Dr Pik said. The 12-month trial, which is being conducted at 20 veterinary clinics around Australia, was launched in December and according to Dr Pik, several dogs had already shown signs of remission. Dr Pik said dogs were given a single dose of chemotherapy — a tablet — that did not target the tumour, but attacked (T-Regulatory) cells within the patient’s own immune system. “If this trial proves effective, we plan to offer this service to canine patients within 24 months and then continue to humans,” Dr Noam Pik
|